Fda Registration Fees 2024
Fda Registration Fees 2024. This fee will be applicable from october 1, 2023, to september 30, 2024.all companies involved in the manufacturing and distribution of medical devices for sale in the u.s. Notable updates to the fee schedule.
Food and drug administration (fda) unveiled the latest medical device user fees and annual establishment registration fees, which are set to take effect. All establishments must pay the establishment registration fee.
Establishments That Are Involved In The Production And Distribution Of Medical Devices Intended For Commercial Distribution In The United States (U.s.) Are Required To Register.
Fda announced fy 2024 (october 1, 2023 through september 30, 2024) fees as follows:
This Fee Will Be Applicable From October 1, 2023, To September 30, 2024.All Companies Involved In The Manufacturing And Distribution Of Medical Devices For Sale In The U.s.
Food and drug administration (fda) has announced new medical device user fee amendments (mdufa) for fiscal year 2024.
The Increase In Fda User Fees From Fy 2023 To Fy 2024 Was 9.5%, Except.
Images References :
 Source: www.libertymanagement.us
Source: www.libertymanagement.us
FDA Registration Renewal Fees Free Renewal Certificate 349, Denovo fees also will increase about 10% to $36,267. Complete form fda 3914 (user fee payment transfer request) to request a transfer of user fees paid to the fda as directed by the food, drug, and cosmetic act (fd&c act).
 Source: www.28ceramics.com
Source: www.28ceramics.com
Best Fda Registration Certificate Manufacture, Fda announced fy 2024 (october 1, 2023 through september 30, 2024) fees as follows: The new 510 (k) for small business is being increased around 10% to $5440.
 Source: www.olympictrading.co
Source: www.olympictrading.co
FDA Certificate, The increase in fda user fees from fy 2023 to fy 2024 was 9.5%, except. There are no waivers or reductions for small.
 Source: www.olympictrading.co
Source: www.olympictrading.co
FDA Certificate, Food and drug administration (fda) issued a federal register notice announcing medical device user fees and annual establishment registration fees. On july 28, 2023, the u.s.
 Source: disqover.agency
Source: disqover.agency
Реєстрація компанії та товарів на FDA Disqover Agency, On july 28, 2023, the u.s. On july 28, 2023, the u.s.
 Source: winnerbiotech.com.tw
Source: winnerbiotech.com.tw
About Winner 萬寧生物科技股份有限公司, Below is the schedule for the 2024 fda fees. The current registration fee for medical devices of all risk.
 Source: eyebanknebraska.org
Source: eyebanknebraska.org
Regulatory Lions Eye Bank, How much did user fees increase for fy 2024? What are the fda user fees for fy 2024?
 Source: blog.naver.com
Source: blog.naver.com
마스크 FDA 등록 방법과 미국 수출 유의사항 네이버 블로그, What are the fda user fees for fy 2024? There are no waivers or reductions for small.
 Source: medicaldeviceacademy.com
Source: medicaldeviceacademy.com
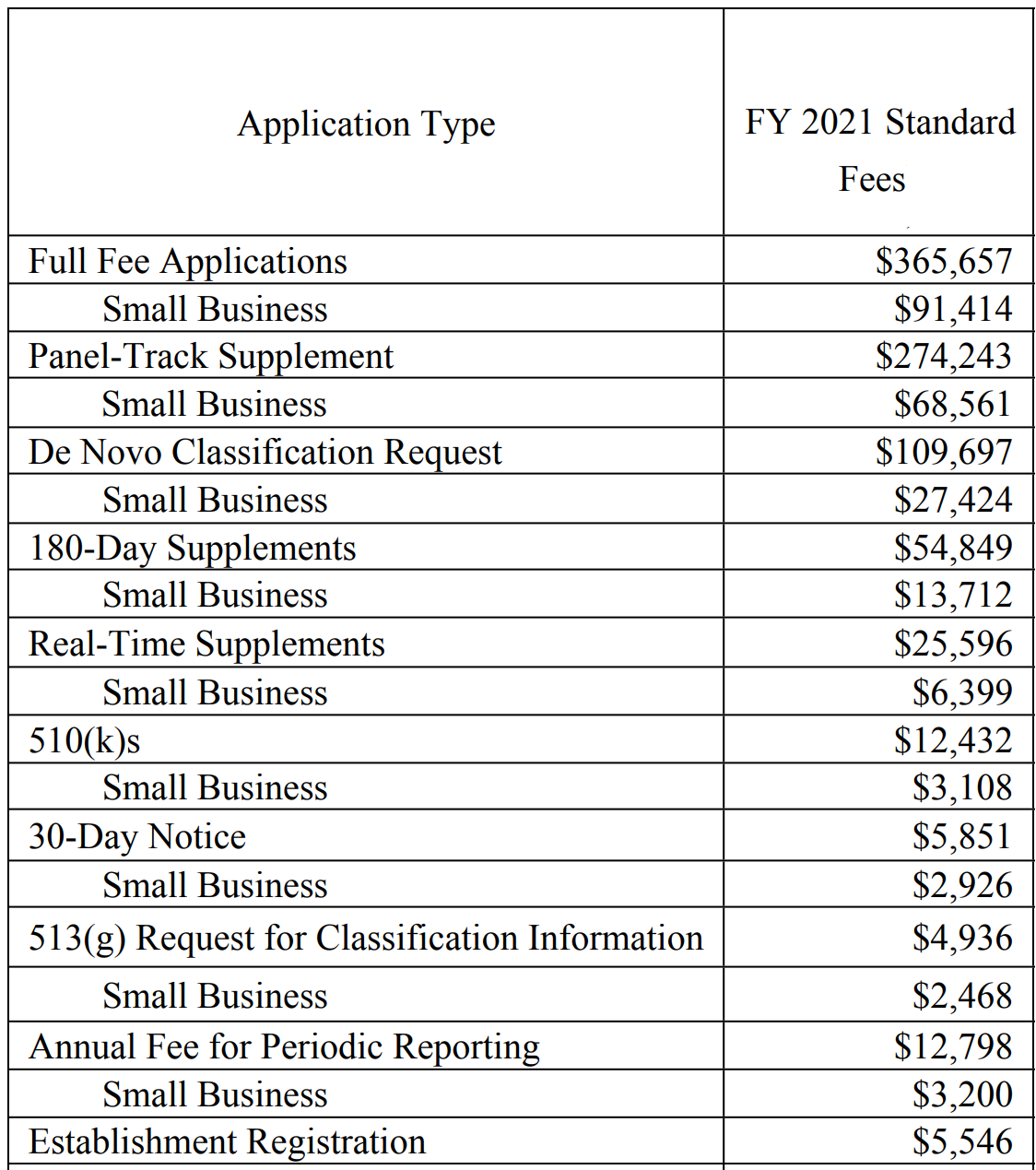
Medical Device 510k submissions, quality systems and training Medical, All establishments must pay the establishment registration fee. Notable updates to the fee schedule.
 Source: www.pinterest.com
Source: www.pinterest.com
Pin on Business Ideas, Notable updates to the fee schedule. What are the fda user fees for fy 2024?
This Fee Will Be Applicable From October 1, 2023, To September 30, 2024.All Companies Involved In The Manufacturing And Distribution Of Medical Devices For Sale In The U.s.
What are the fda user fees for fy 2024?
First, The Proposed Price Increases Will Be Significant.
All establishments must pay the establishment registration fee.